(1) Why do cyclohexane disubstituted compounds have cis-trans configurations? We know that the predominant conformation of cyclohexane is chair conformation, and it can be converted from one chair conformation to another through ring rotation. Choose one of the chair conformations and observe carefully, you can find that there are three upright bonds (a) and three flat bonds (e), and three downright bonds (a) and three flat bonds (e).
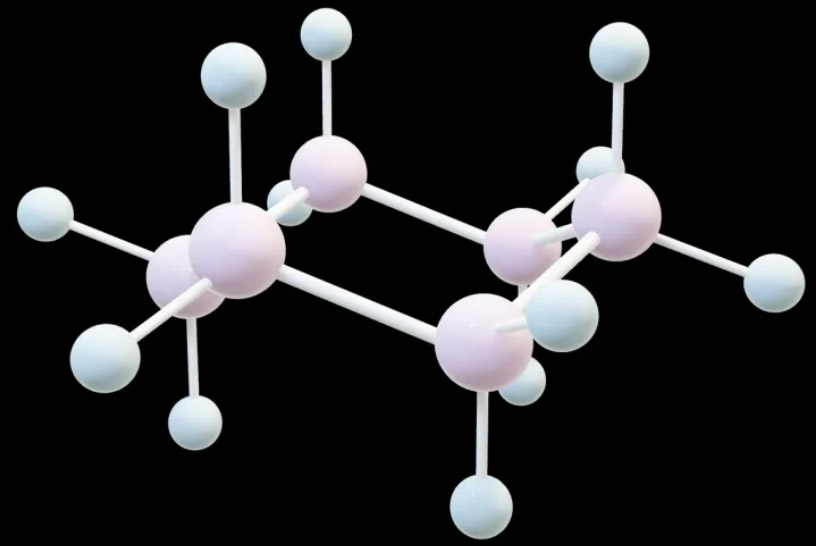
Although the six carbon atoms on cyclohexane are not completely in one plane, they can be considered to be approximately in one plane, and there are 3a and 3e above and below the plane.
During the swivel process from one chair conformation to another chair conformation, the up a bond becomes an up e bond, and the down e bond becomes a down a bond (please watch the video below , stare at one of the hydrogen atoms, and then change the other one. Note: the swirl effect does not necessarily reflect the microscopic world completely, but only approximates).
However, no matter how it changes, the atoms on the surface are always on the surface, and the atoms below the surface are always on the surface, and the rotation of the carbon-carbon bond will not change the fact that they are separated on the surface and the surface. Therefore, when there are substituents on two different carbons of cyclohexane, there will be two configurations of “group on the same side of the plane” and “group on the different side of the plane”, that is, cis-trans configuration. The transition between the two configurations can only be achieved through bond breaking and reconnection, but not through swirl. In other words, although the carbon-carbon single bond of cyclohexane can rotate, it is not completely free, but moves cooperatively, so that the substituents located on different sides of the plane cannot be located on the same side through rotation.
(2) Determination of the dominant conformations of various configurations of cyclohexane disubstituted products
(taking dimethylcyclohexane as an example) First of all, it is necessary to clarify: the parent ring of the dominant conformations of various configurations of dimethylcyclohexane Both must be in chair conformation. However, under the action of swivel, the two chair conformations will transform each other, which will lead to the transformation of a bond and e bond between the two substituents. The dominant conformation we want to determine is to choose between these two typical conformations before and after the rotation. And, when we write different configurations, we must choose the most stable one to write.
So how can you tell which conformation is stable? If the substituent appears on the a bond, it is very close to the hydrogen atom or group in the meta position, and there will be a strong repulsion force, which will inevitably lead to a decrease in structural stability, so more and larger substitutions should be made. Based on the e bond, the conformation will be stable.
Tips: The text content comes from the Internet
Extended information
Visit Cyclohexane Wikipedia
Visit the Cyclohexane product page
How to understand the half-chair conformation of cyclohexane?
How many carbon atoms are coplanar in cyclohexane?

